C-DOCTOR ITP Program
C-DOCTOR maintains a portfolio of Interdisciplinary Translational Projects (ITP) teams that have been selected based on their relevance in addressing unmet clinical needs in the dental, oral, and craniofacial fields through a regenerative mechanism. ITP teams are developing technologies that align with our clinical indication priorities in dental, oral, and craniofacial tissue engineering/regenerative medicine (TE/RM).
ITP teams recruited from universities and industry nationwide demonstrate strong market potential, high anticipated patient value, and high probability of clinical adoption. C-DOCTOR’s pivotal role in ITP teams prioritizes advancing clinical adoption and commercial viability by scaling-up and validating manufacturing, confirming their regulatory path and clinical trial plans, and establishing commercial partnerships.
Dental, Oral, & Craniofacial
Tissues Targeted by ITPs
C-DOCTOR aims to select ITP teams that are refining tissue engineering/regenerative medicine products/technologies that address unmet clinical needs in the following dental, oral, and craniofacial tissues.
Bone
Skin

Muscle
Salivary gland
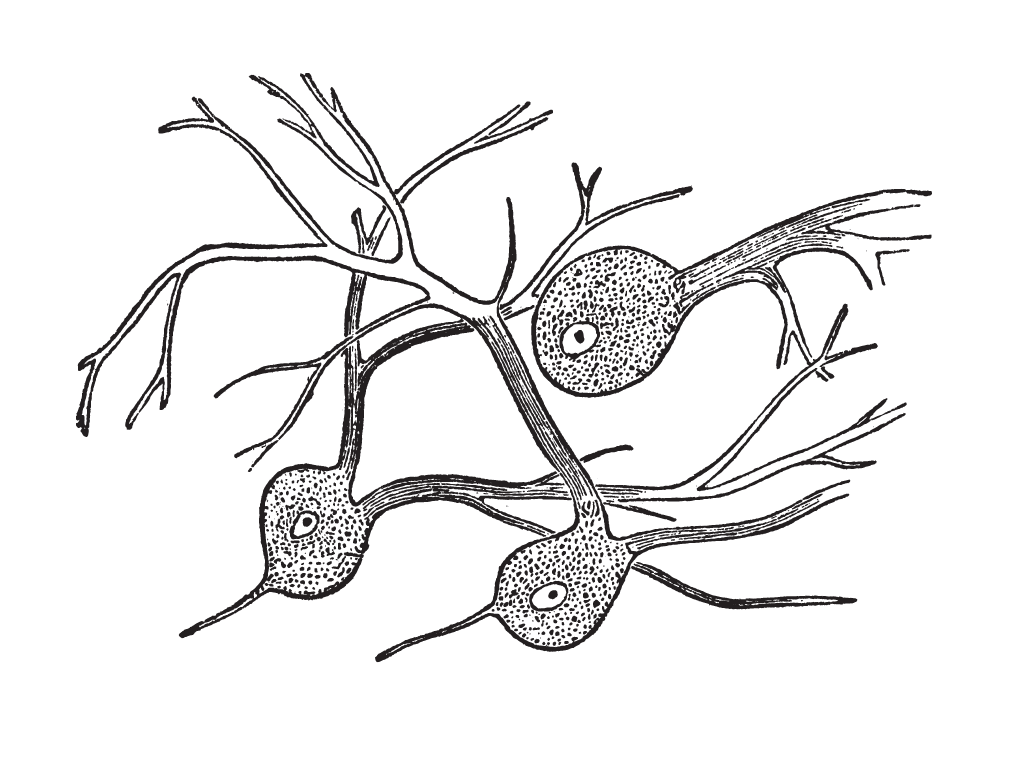
Nerve

Periodontium

Fat
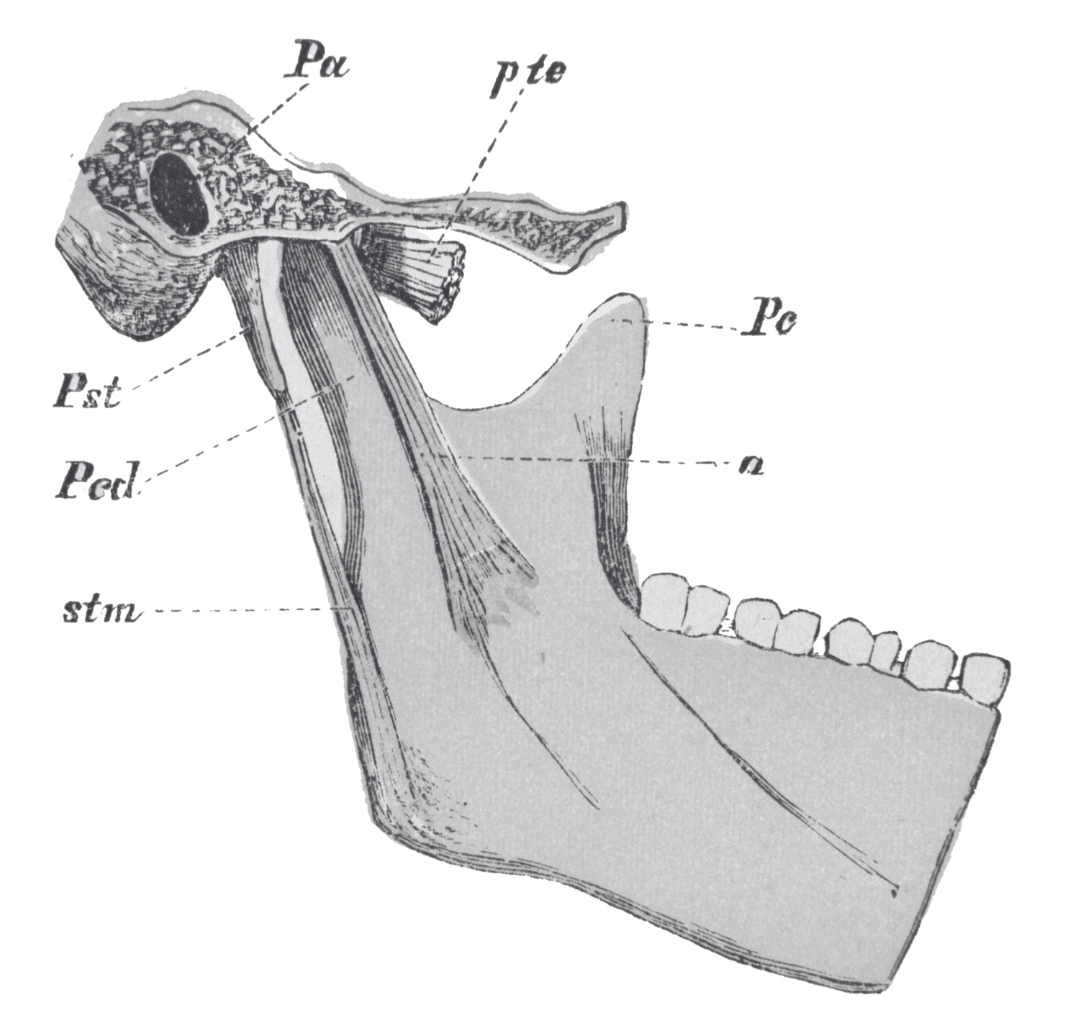
TMJ
In addition to funding support...
ITP teams are closely supported by C-DOCTOR resources such as a customized advisory board, industry-experienced project managers, C-DOCTOR partner institutions, and advisor networks. These resources help to further the ITP teams’ translational activities: pre-clinical scientific studies, protocol/technical methodology development, commercialization strategy, and regulatory interactions supporting IDE/IND submission and associated clinical study protocols.
The End Goal for ITPs
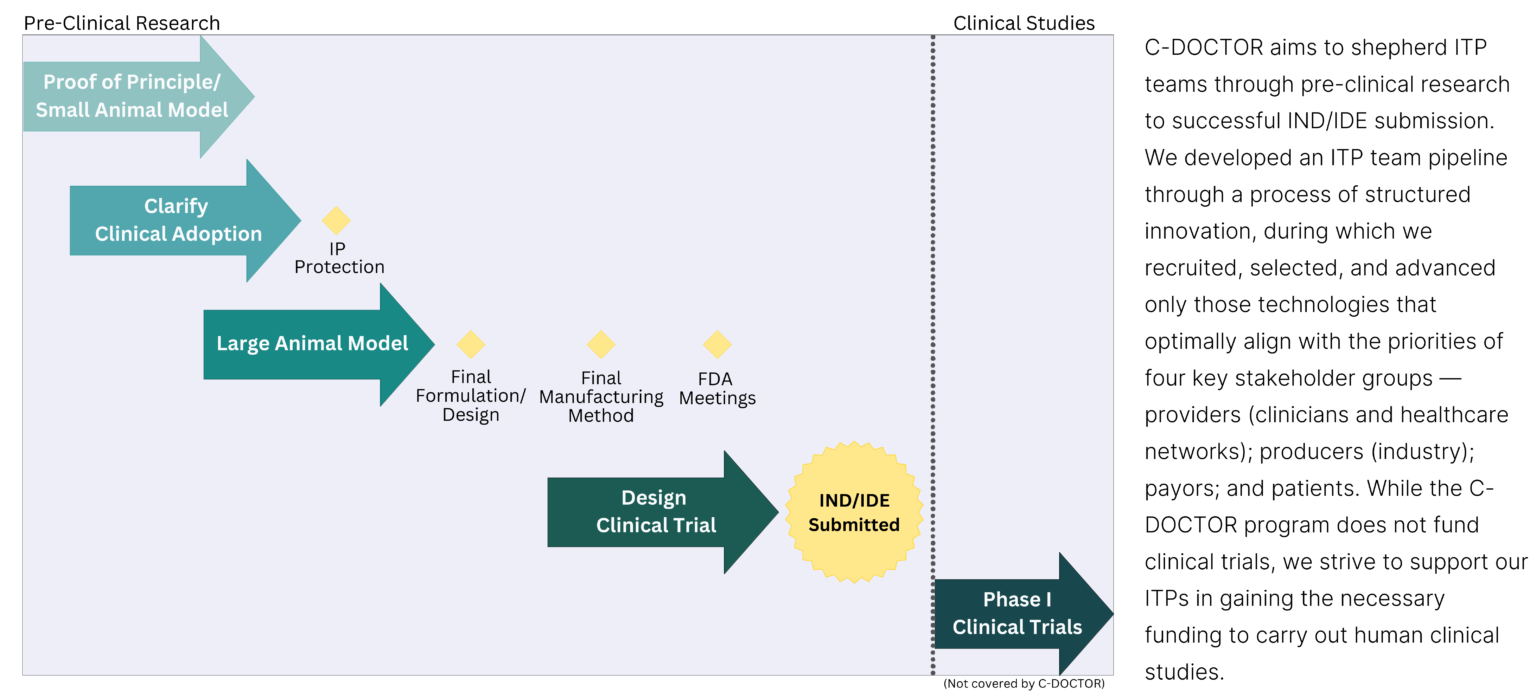
ITP Criteria
ITP proposals are reviewed using the following criteria
Significance
Technology/product uses a regenerative mechanism, addresses a significant unmet clinical need, taps into a total addressable market, and demonstrates potential for clinical adoption.
Innovation and Impact
Technology/product demonstrates advanced stage of technology, often with early proof-of-concept data, and in-vivo evidence of efficacy for a craniofacial indication.

Competition
Technology/product demonstrates unparalleled competitive advantage with existing treatments and technology and has a patent or potential for patent filing.
Work Plan Strategy and Aims
Technology/product demonstrates effective pre-clinical development strategy with commercialization potential.
ITP Selection Process
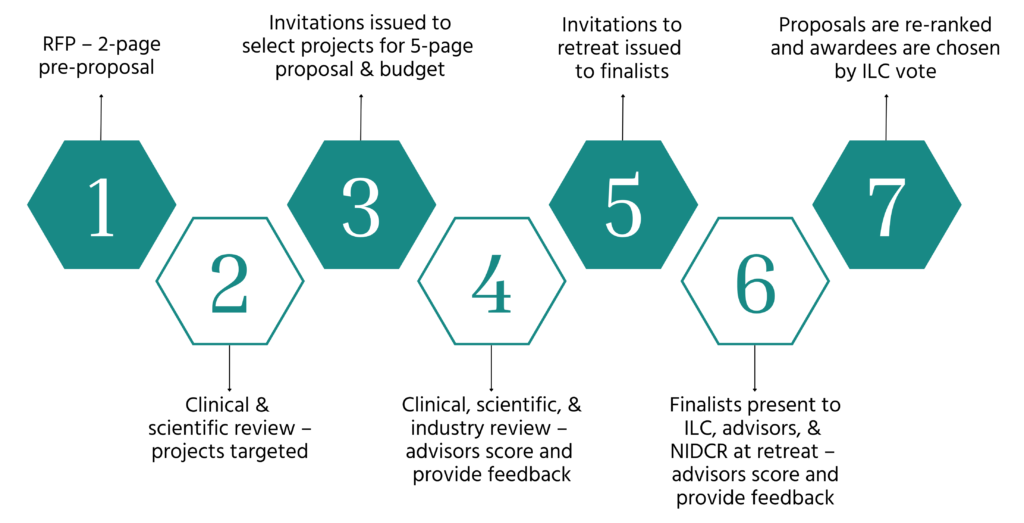
ITP IND/IDE-Enabling Activities
Research/Technical
Research/Technical
Commercialization
Regulatory Interactions
Regulatory Interactions
Manufacturing
Manufacturing
“C-DOCTOR has provided us with expertise in early-stage drug-device development and commercialization of drug products. Dr. Michael Jamieson has been a valuable resource to us for discussions regarding FDA IND applications, negotiating licensing agreements with the manufacturer of our pharmacological agent and other regulatory aspects of our C-DOCTOR project.”
– Geoffrey Gurtner, MD (ITP PI)
Click here to learn more: Scar Formation Prevention
Meet our
ITP Teams
ITP Program Benefits
The support that the C-DOCTOR network and its nationwide collaborative infrastructure provide to ITP include, but are not limited to team coaching, guidance documents, outreach/training opportunities and assistance with the following:
Development of translational-oriented milestone development
Identification of potential partners for clinical trial funding and execution



Implementation of design control processes
ITP Program
Request for Proposals (RFP) Cycles: 5
Proposals Reviewed: 119
ITPs Awarded: 22
Active ITPs: 9
As of August 2023
C-DOCTOR is currently not accepting proposals for new ITPs. Please check later for future Request for Proposals.
If you have any questions, please email VyVy Nguyen. Thank you!
C-DOCTOR
A national resource center for the clinical translation of innovative regenerative technologies to replace dental, oral, and craniofacial (DOC) tissues or organs lost to congenital disorders, traumatic injuries, diseases, and medical procedures.
Contact Us
- Jeffrey Lotz, Co-PI
- Yang Chai, Co-PI
- Pui Yee Law, Operations
- Bridget Samuels, Operations
- VyVy Nguyen, Operations
C-DOCTOR is supported by U24 DE029463 from the National Institute of Dental & Craniofacial Research, National Institutes of Health.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.