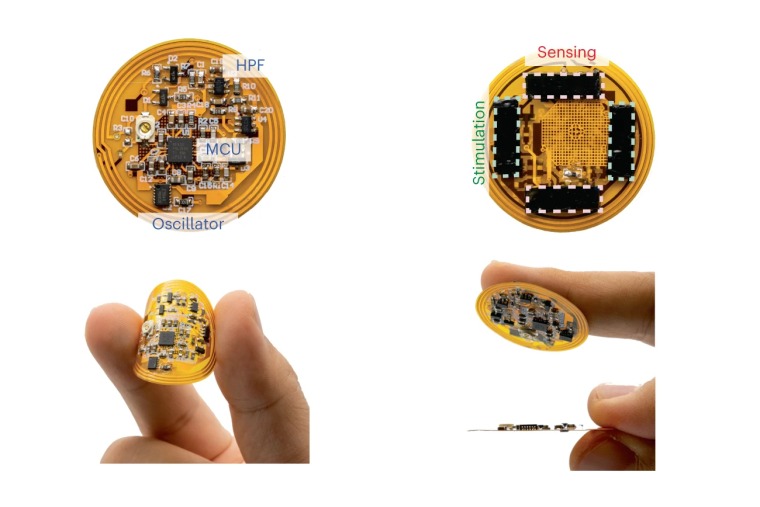
The 2024 C-DOCTOR Spring Retreat was hosted at the Residence Inn by Marriott at Berkeley, CA on June 3–4, 2024. The Center for Dental, Oral, & Craniofacial Tissue & Organ Regeneration (C-DOCTOR) is comprised of nine Interdisciplinary Translational Projects (ITP) teams, one having a recent successful IND filing and making efforts to begin a first-in human trial. This year’s C-DOCTOR retreat was supported in part by the California Institute for Regenerative Medicine (CIRM) through conference grant: EDUC1-16388. The retreat was an excellent opportunity for attendees to connect with other project teams, the C-DOCTOR leadership, and external advisors and consultants to network and collaborate. The event highlighted the progress of the projects in C-DOCTOR’s ITP portfolio. C-DOCTOR celebrated the progress of its ITPs with five ITP teams with eight FDA interactions (e.g., IND submission, Pre-Submissions and clarification teleconferences, and Requests for Designation). In addition to updates from the ITP teams, the retreat featured a variety of sessions and panels to demonstrate C-DOCTOR’s progress to date and opportunities to grow in the future. A summary of the sessions is below. CIRM Presentation Lisa Kadyk, PhD (Associate Director of Therapeutics Development) gave a presentation about CIRM’s mission, goals, and funding source; the different grant mechanisms available through CIRM; and non-grant resources available through or associated with CIRM. Sensors and Wearable Diagnostics Session: Clinical Applications and Use Cases in the DOC Space and their Role in Tissue Regeneration Geoffrey Gurtner, MD and Uttam Sinha, MD, gave presentations on the development of sensors or wearable diagnostics products and their clinical applications. Dr. Gurtner presented on the development of his smart bandage (Click here to read more). Dr. Sinha presented on his SaliSense technology aimed at early detection of head and neck cancer. These presentations served as the baseline for discussion to explore the role of these products in the future direction of the C-DOCTOR program. Best Practices for FDA Interactions Focusing on IND/IDE-Enabling Data: Experts from CDRH and CBER Srinivas Nandkumar, PhD, Adam Chin, PhD, Sherrill Lathrop Blitzer, PhD (FDA CDRH), and Mondona McCann, PhD (FDA CBER) presented on best practices for FDA interactions with a focus on IND/IDE-enabling data such as determining appropriate animal models, preparing data for the FDA, as well as common challenges/pitfalls during FDA interactions and how to avoid them. This session was timely as more ITPs in the C-DOCTOR portfolio plan for FDA interactions in the upcoming year in the form of full IND/IDE submissions, Pre-Submissions, and Requests for Designations. Bridging Academic Regulatory Science Centers and C-DOCTOR for Enhanced Regulatory Science Practice and Training Kathy Giacomini, PhD, BSPharm and Eunjoo Pacifici, PharmD, PhD presented on the UCSF-Stanford Center of Excellence in Regulatory Science and Innovation (CERSI) and the training programs available at the USC School of Pharmacy’s Department of Regulatory and Quality Sciences. They provided discussion on how C-DOCTOR can create further collaborations with organizations like the FDA and improve internal C-DOCTOR practices. C-DOCTOR and Looking Ahead: Integrative Health Helene Langevin, MD presented on the mission and current initiatives at NIH RE-JOIN and provided an overview of musculoskeletal integrative health. Her presentation served as a springboard for how to understand and leverage mutual synergies between C-DOCTOR, NIDCR, and other NIH institutes. C-DOCTOR receives funding from the National Institute of Dental and Craniofacial Research (NIDCR; award: U24DE029463) with the mission to shepherd new therapies through pre-clinical studies and into human clinical trials, commercialization, and broad clinical adoption. As C-DOCTOR enters year 5 of its funding cycle, it looks forward to achieving continued success with more IND/IDE filings and more FDA interactions. In addition, C-DOCTOR poises itself to be at the forefront of product development and innovation in tissue engineering/regenerative medicine in the dental, oral, and craniofacial field and beyond.